Colored light is exactly the same kind of radiation as X-rays, gamma rays, UV rays, radio waves, or microwaves. The only difference is in its frequency (the number of times the wave can oscillate between two maxima in a second) and wavelength (the distance between two maxima). Other than that, it's all the same thing. We call light having a wavelength of about 400-700nm (100nm = 10-7m) visible light only because it ends up that our eyes process it when it hits them. But all of it is light. So what's going on that makes us see certain wavelengths as color?
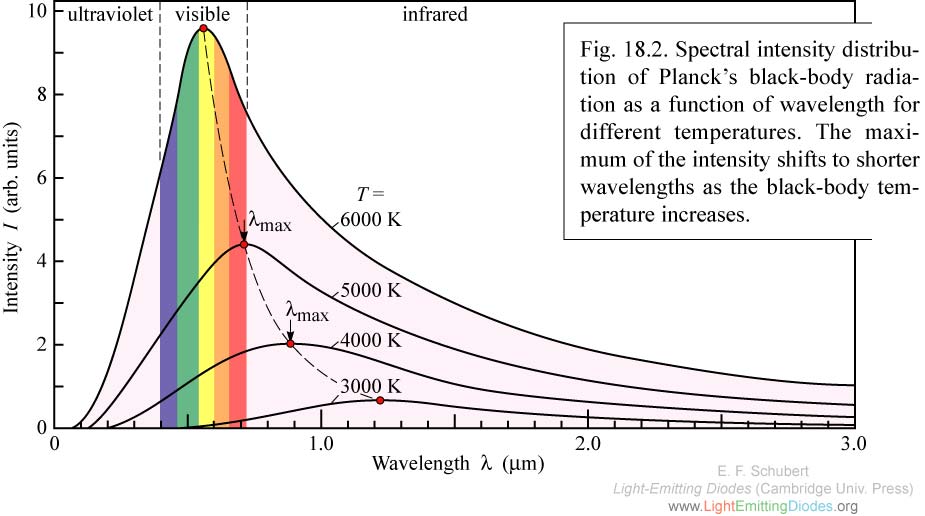 First off, why do objects give off some wavelengths of light but not others? There are two ways an object can have (or lack) color. First, an object can emit light all by itself. You don't do this in the visible range, but the stars do. The graph here shows the light output of several different kinds of stars. You'll notice that each star emits light in all of the visible colors, but in one more than all the others. That's why some stars look blue and others red. Ours looks like the middle curve and actually appears white in space (emitting all of the colors fairly evenly) although it appears yellow on earth (we'll get to that in a bit).
First off, why do objects give off some wavelengths of light but not others? There are two ways an object can have (or lack) color. First, an object can emit light all by itself. You don't do this in the visible range, but the stars do. The graph here shows the light output of several different kinds of stars. You'll notice that each star emits light in all of the visible colors, but in one more than all the others. That's why some stars look blue and others red. Ours looks like the middle curve and actually appears white in space (emitting all of the colors fairly evenly) although it appears yellow on earth (we'll get to that in a bit).The next way an object can have color is by scattering light that is incident upon it. Depending on the chemical composition of the material, it will absorb some wavelengths and scatter others. Obviously, only the wavelengths that get to your eye are the ones that your brain processes, so you perceive distinct colors in objects illuminated with white light. Scattering is a rather prevalent phenomenon. One of the most common occurrences happens with white sunlight traveling through our atmosphere. It just so happens that the size of air molecules corresponds very well to scattering smaller wavelengths of light. Blue, having the smallest wavelength in visible, is preferentially scattered in every direction, which is the reason we see it when we look at any part of the daytime sky (this is called Rayleigh scattering). If we looked at the source of the light, the sun (note: do not look at the sun), we would expect to see the remaining light; white minus blue, which we call yellow. In other words, if our sky scattered red light, our sun would look green instead. Particles much larger than molecular gas particles (such as water vapor particles) scatter light, but do so evenly. Clouds (composed of water vapor) thus scatter all incident light that they receive evenly, causing us to see white (a phenomenon called Mie scattering).
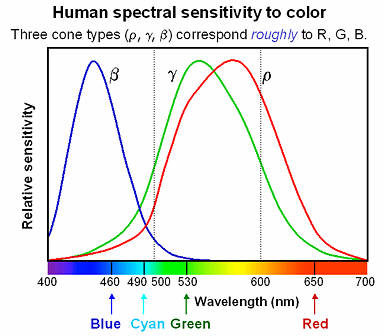 So, when (scattered or emitted) light reaches our eyes, how does our brain distinguish between all the colors? As you are well aware, our eyes have four kinds of small photoreceptors in them called generally rods and cones. Each, by a process known as phototransduction, transmits electrical impulses to the brain when hit with light. However, not all of them respond to the same wavelengths. Some only respond to blue, and others only to green or red. The graph here displays the response functions by wavelength of the three different kind of cones in our eyes. You see that one transduces primarily in the blue range whereas there are two that transduce in almost the same range, but one slightly redder than the other.
So, when (scattered or emitted) light reaches our eyes, how does our brain distinguish between all the colors? As you are well aware, our eyes have four kinds of small photoreceptors in them called generally rods and cones. Each, by a process known as phototransduction, transmits electrical impulses to the brain when hit with light. However, not all of them respond to the same wavelengths. Some only respond to blue, and others only to green or red. The graph here displays the response functions by wavelength of the three different kind of cones in our eyes. You see that one transduces primarily in the blue range whereas there are two that transduce in almost the same range, but one slightly redder than the other.Color, then, is just the end product of our eyes' response to a source. Imagine a source at 450nm. The blue receptor responds strongly and green and red each respond to a much lesser degree, but green a little more than red. Thus we see mostly blue with a much smaller dose of red and green. In other words, we see blue on its way to becoming purple.
 Looking at the response graph, one can deduce that the easiest color to see is at almost exactly 550nm. Here, red and green respond equally in strong measure, producing a sickly-yellow color. It as at this intersection point where the largest number of photoreceptors are giving some kind of response. Interestingly, a human's ability to see this color so well is the reason that they started painting emergency vehicles this color (as pictured here).
Looking at the response graph, one can deduce that the easiest color to see is at almost exactly 550nm. Here, red and green respond equally in strong measure, producing a sickly-yellow color. It as at this intersection point where the largest number of photoreceptors are giving some kind of response. Interestingly, a human's ability to see this color so well is the reason that they started painting emergency vehicles this color (as pictured here).All of the colors that we see are simply combinations of red, green, and blue. Sometimes they are represented in the form <ratio of red, ratio of green, ratio of blue>. The "pure" colors are ones that can be represented by only one wavelength. In other words, if you can produce a color by drawing a single vertical line on the receptor graph above and mix the resulting ratios of red, green, and blue, you are seeing what a "pure" color. Some colors require that at least two wavelengths of light combine to create the response in our eye. Brown is the most common example. Consequently, that's why brown is not part of the rainbow; a rainbow diffracts light and allows you to see white light (a combination of all colors) split up into single wavelength portions. Since brown cannot be created in the human brain without at least two stimuli, it cannot be in the rainbow.
The science behind scattering, absorption, and reflection is much, much deeper. But I hope that this allows at least the first look into the beautiful complexity of optics and biology as an application of physics (of course). The resolution of our eyes is astounding. The difference between blue and red light (the extremes of our vision) is only about 10-7m yet our eyes distinguish the myriad of colors and details that make our world vibrant and beautiful.
WOW! I wondered why fire trucks were being painted a different color! I had never thought about why brown wasnt in the rainbow either -- very interesting!
ReplyDeleteTHANKS!